In order to assess the sustainability of algal biofuel, one needs to have an understanding of the individual components that compose its potential supply chains. Here, we will focus on the basic processes involved in biofuel production – from the traits and biology of the organisms, to cultivation methods, and to process it into liquid fuels. We will also focus on algal strains and its different attributes vital for biofuel production, the photoautotrophic method for algae cultivation through closed photobioreactor and open-pond systems, the dewatering and collection processes, and the processing of biomass, algal lipid, or secreted products into fuels.
In order to assess the sustainability of algal biofuel, one needs to have an understanding of the individual components that compose its potential supply chains. Here, we will focus on the basic processes involved in biofuel production – from the traits and biology of the organisms, to cultivation methods, and to process it into liquid fuels. We will also focus on algal strains and its different attributes vital for biofuel production, the photoautotrophic method for algae cultivation through closed photobioreactor and open-pond systems, the dewatering and collection processes, and the processing of biomass, algal lipid, or secreted products into fuels.
-
Algal Feedstock
The different organisms that can be used as potential feedstock for algal biofuel production belong to a diverse collection of aquatic organisms that perform oxygen-evolving photosynthesis. They also lack the roots, embryos, leaves, and stems of plants (Leliaert et al., 2011). This category is also comprised of eukaryotic species related to this plant lineage. It can be further considered as microalgae (microscopic species) or macroalgae (large structured species).
In terms of biofuel, the term microalgae are also comprised of cyanobacteria which is a diverse prokaryotic lineage whose earlier variation resulted in the plant chloroplast (Keeling, 2010). McKenzie (2011) further assessed that eukaryotic and prokaryotic microalgae are responsible for over 40% of the net primary productivity on the planet. When it comes to biofuel feedstock, algae is a more appealing option compared to land plants due to its faster biomass which can double the cycle. It also has a more accessible form of stored carbon compared to the lignocelluloses used for the production of cellulosic biofuels. Algae is also a more appealing option due to its ability to thrive on land sites and water sources that are not suitable for terrestrial farming.
Furthermore, did you know that microalgae contain diverse metabolites and pigments that can be used as colorants and nutritional supplements? Some examples of such products include a high-protein powder which is a derivative from cyanobacterial species of Spirulina (Arthrospira), an antioxidant derivative from the alga Haematococcus, and astaxanthin (Gershwin, 2008; Guedes et al., 2011). For more than a decade, there have been several commercial-scale algal ponds that grow these and other microalgae.
On the other hand, the required scale of deployment for fuel algae cultivation is expected to be larger compared to the cultivation of nutraceuticals and other specialty products. After all, producing biofuel from algae necessitates expanding and exploiting the demonstrated commercial-scale growth of algal biomass. Moreover, it also requires harvesting the relatively accessible carbon stored within. Carbon is stored within algal cells in several forms, and using different technologies, these molecules can be accessed. Carbon is such a vital element for algae that it is typical for them to store excess carbon when a cellular division is constrained by some factor other than carbon availability—this situation is termed unbalanced growth. In several eukaryotic microalgae, photosynthetic carbon fixation carries on despite unbalanced conditions.
 Under protracted periods of environmental stress, the surplus fixed carbon is deposited in the form of neutral lipids known as triacylglycerols (TAGs). Essentially, TAGS are hydrocarbon chains that are terminated in a carboxylic acid group. Furthermore, the three carboxyl groups are connected to glycerol through an ester linkage. Biofuels that contain hydrocarbon chains that are longer than six carbons are especially valued because of their volatility, high heats of combustion, and compatibility with existing engines.
Under protracted periods of environmental stress, the surplus fixed carbon is deposited in the form of neutral lipids known as triacylglycerols (TAGs). Essentially, TAGS are hydrocarbon chains that are terminated in a carboxylic acid group. Furthermore, the three carboxyl groups are connected to glycerol through an ester linkage. Biofuels that contain hydrocarbon chains that are longer than six carbons are especially valued because of their volatility, high heats of combustion, and compatibility with existing engines.
Extracted TAGs can be converted into biodiesel through the use of different technologies such as hydrotreating and transesterification. However, algal species that doesn’t store large TAG amounts can still be converted to biofuels by using different chemical conversion technologies. For instance, some species that contain polysaccharides can be fermented to produce ethanol.
From its early foundations of algae cultivation for human nutraceuticals and fish feedstuff, the algal biofuel industry is vastly emerging and evolving. Choices of algal feedstocks have been increasing in order to meet the goals of fuel production, rather than nutritional content. New technologies are also utilized for processing biomass that goes beyond those that focus on TAGs. Ideal attributes for algal feedstock for fuels include efficient use of light, nutrients, and carbon dioxide (CO2) under a variety of temperatures; rapid and dense growth; buildup of desirable macromolecules that can be converted into fuels; resistance to pests and predators; absence of unwanted by-products; and ease of harvest.
In the United States, research and commercial interest have focused on microalgae. After all, some reports show that microalgae have research short-term maximum productivities of 50-60g dry weight per square meter per day in a CO2-enriched open pond in California and Hawaii (Sheehan et al., 1998). This study, along with other reports of microalgae’s productivity has promoted its reputation as a prime candidate for providing cheap biomass feedstocks that can be used for feedstuff, food, or energy.
-
Diversity of Strains
When choosing strains for biomass production, one must consider what their desired product is, and what technology will be used for the production of fuel. One must also consider the type of cultivation facility (closed vs. open) and the source. Some experiments using outdoor ponds focused on the production of biodiesel through transesterification of TAGs in order to yield fatty-acid methyl esters (FAME). Here, strains that amass TAGs were chosen.
Five different groups of microalgae were categorized as the high priority for biofuel production by the U.S. Aquatic Species Program (Sheehan et al., 1998). These five categories are green algae (Chlorophyceae), eustigmatophytes (Eustigmatophyceae), diatoms (Bacillariophyceae), prymnesiophytes or haptophytes (including Prymnesiophyceae), and golden-brown algae (Chrysophyceae). Numerous strains of eukaryotic microalgae are also probable high-oil producers for large-scale culture (Sheehan et al., 1998; Rodolfi et al., 2009). These include species of Chlorococcum, Chlorella, Dunaliella, Tetraselmis, Scenedesmus, and particularly Botryococcus braunii and Neochloris oleoabundans from Chlorophyta; the genre of Amphora, Cylindrotheca, Navicula, and Amphiprora, and the species of Phaeodactylum tricornutum, Chaetoceros muelleri, Nitzschia dissipata from Bacillariophyta; the species of N. salina and Nannochloropsis ocalata from Eustigmatophyceae; and the genera of Pavlova and Isochrysis from Haptophyta.
The scope of organisms that are being considered for biofuel production are being changed due to the improvements of several technologies that can covert total biomass to produce drop-in fuels. These includes technologies such as Xtrudx (Xtrudx Technologies, 2012), Inventure (Inventure, 2012), and Solvent Rescue Limited (Solvent Rescue Limited, 2012). Academic institutions like Old Dominion University (Hatcher, 2011) are part of the change as well.
All classifications of algae are rich in polar lipids and they can be recovered by such processes. They also contain cellulose and other polysaccharide cell walls made up of sugars. Cyanobacteria store extra carbon as glycogen instead of TAGs, and cyanobacteria and macroalgae amass quantities of other complex polysaccharides. If appropriate processing technologies are available, these and several other macromolecules, are all probable carbon sources for the production of drop-in fuels. Moreover, algal carbohydrate can be used as a feedstock for fermentative fuel production procedures based on heterotrophic organisms, like those used by Solazyme (Solazyme, 2012) and LS9, Inc. (LS9 Inc., 2011). On the other hand, Algenol uses cyanobacteria for ethanol production (Chance et al., 2011a; Algenol Biofuels, 2012). As of 2012, several marine macroalgal species are being considered in the production of biofuel in India.
 The red algal species Kappaphycus alvarezii is a species that is cultivated for its high carrageenan content (Russell, 1983; Rodgers and Cox, 1999; Woo et al., 2000). Spirulina species are suitable for aquaculture and they are grown at large scales which are then sold as a nutritional supplement (Earthrise Nutritional, 2009a). However, the range of cyanobacteria that is suitable for fuel production is still mostly unexplored. Prokaryotic algal species deliver additional diversity in tolerance of growth habitat and pH, light harvesting, and facility of genetic modification. Additionally, some cyanobacterial species are diazotrophs which means that they are able to repair atmospheric nitrogen (N). While there are no existing commercial operations that rely on a nitrogen-fixing strain, numerous filamentous strains with good light harvesting properties and for which genetic methods are well developed are diazotrophic (Heidorn et al., 2011; Ruffing, 2011). The utilization of these strains as a nitrogen provider or as a biofuel feedstock for non-fixing strains (to reduce nutrient input) has garnered little attention.
The red algal species Kappaphycus alvarezii is a species that is cultivated for its high carrageenan content (Russell, 1983; Rodgers and Cox, 1999; Woo et al., 2000). Spirulina species are suitable for aquaculture and they are grown at large scales which are then sold as a nutritional supplement (Earthrise Nutritional, 2009a). However, the range of cyanobacteria that is suitable for fuel production is still mostly unexplored. Prokaryotic algal species deliver additional diversity in tolerance of growth habitat and pH, light harvesting, and facility of genetic modification. Additionally, some cyanobacterial species are diazotrophs which means that they are able to repair atmospheric nitrogen (N). While there are no existing commercial operations that rely on a nitrogen-fixing strain, numerous filamentous strains with good light harvesting properties and for which genetic methods are well developed are diazotrophic (Heidorn et al., 2011; Ruffing, 2011). The utilization of these strains as a nitrogen provider or as a biofuel feedstock for non-fixing strains (to reduce nutrient input) has garnered little attention.
Significant differences are present in carbon storage forms (important as fuel feedstock), accessory pigments such as carotenoids (which can be valuable commercial products), and dominant pigments (important for solar energy capture) among different algal division. Additionally, their composition and pigmentation are affected by environmental stress and growth conditions.
A recent study shows that increased lipid production in algal cultures as a function of species ranges in mixed cultures under nutrient-limiting growth circumstances (Stockenreiter et al., 2012). However, this effect has only been demonstrated in low-density natural algal populations or in a laboratory scale and it necessitates confirmation at relevant volumes and extended periods of time. Additionally, lipid production of mixed algal culture might vary under the nutrient-replete circumstances of ponds designed for maximum growth. Mixed cultures might facilitate diversity of products through product conversion, efficient use of light in the water column, flocculation and harvesting improvements, and cross-protection (Stomp et al., 2007). However, mixed cultures increase the heterogeneity of the prospective product, which may affect the quality of yield and the ability to enhance the varied characteristics of the mixture for one product. The perspective to enhance the algal biofuel supply chain through the growth of mixed cultures merit additional research to determine its effects on desired product yield and biomass accumulation.
The difficulty of converting desirable strain properties from the lab to the field is one of the biggest challenges for strain selection. A desirable strain must have robust growth in open ponds under natural cultivation and weather conditions, and it must retain qualities that are measured and selected in controlled laboratory conditions. However, the ability to compete and grow well when it is exposed to environmental conditions can be hard to predict. Few strains have already proven to be healthy in outdoor mass cultivation, however, years of investment in process and time went into their commercial development.
-
Desirable Strain Properties
Notwithstanding its strain type or technology used, the vital goal is to maximize the quantity of a final product per unit area, time, or water volume. Additionally, the goal is to make the most of the product per nutrients, unit input of energy, and other resources. Lipid accumulation and biomass per unit time are two measures of productivity. There are several important conditions when selecting algal strains for commercial biofuel production. This includes considering variables that can alter cost in the supply chain that are crucial for economic viability (for example, AQUAFUEL, 2009). Preferably, the criteria for strain selection must be measurable. The important selection criteria include:
- Photosynthetic Efficiency. Photosynthetic efficiency is the most objective measure when comparing the productivity of algae with land crops. Basically, it is defined as the percentage of available light (energy) that is transformed into biomass energy.
- Harvestability. Energy consumption and harvesting costs can differ significantly between different algal strains (Uduman et al., 2010). Some factors that contribute to these differences include the capability for induced bioflocculation or auto-flocculation and sedimentation rate. Species with positive buoyancy, filamentous strains that can be seined, or species that settle out of the water column rapidly once agitation stops may not necessitate centrifugation, and they can be easily harvested. Growing mat-forming algal films or algae can facilitate harvesting (Tang et al., 1997), but to the researcher’s knowledge, such methods have not been scaled up. Methods that depend on harvesting secreted products rather than biomass simplify the harvesting process, but such methods need photobioreactors for algae cultivation in order to avert contamination by microorganisms that would consume the product.
- Non-Toxic. A variety of non-toxic algal strains can increase its social acceptability. Furthermore, it can also lessen the potential impact of accidental releases and occupational exposures.
- Extractability and processability. These parameters include factors that can influence the process of algal biomass to fuels and ease of algal oil extraction. For instance, thickness, presence of tough fibers, cell volume, moisture content, and cell wall toughness (Brennan and Owende, 2010).
- Robustness. Robustness refers to the total stability of the crop. This depends on its resistance to environmental variables and climate (e.g. pathogens and predators, temperature, dissolved solutes and salinity, and pH). Microalgae strains have different tolerances to these variables. Moreover, a strain’s ability to flourish in water with various metals, salts, and other solutes may become increasingly vital as competition for freshwater use between different segments increase. Resistance to high pH allows a strain to grow in alkaline conditions that favor a monoculture crop instead of sensitive pathogens and predators. Species with large cell sizes or filamentous species tend to be more resilient against unicellular species with small cell sizes (Tillmann, 2004). Being tolerant to different temperature conditions can be crucial if the algae are cultivated in areas with seasonal temperature fluctuations. In order to maintain year-round production, it is recommended to rotate strains with different temperature tolerance profiles. Robustness can be evaluated by scoring the strain success under different potentially relevant conditions such as in Evens and Niedz (2011).
- Local Origin. Locally harvested strains can improve sustainability and ease management (RSB, 2011). In fact, some governments have restricted the importation of nonnative specifies (e.g. the 81st Texas Legislature House Bill 3391 of 2009). On the other hand, the wind-borne and diverse nature of algae makes it unlikely that legislation can define its species as nonnative or native. Regardless of legislation, local strains can have unique adaptations depending on the local water, climate, and parasites that laboratory-grown or imported strains may not have.
- Value of Coproducts. Algal biomass can be used to yield other significant coproducts. This includes products like docosahexaenoic acid, phycobilins, eicosapentaenoic acid, or carotenoids, (Pal et al., 2011). Coproducts may counterbalance some of the costs of the biofuel product. However, please note that the market value of coproducts may decrease under a high-demand and low-demand condition.
-
Strain Engineering and Development
Presently, our modern agriculture has achieved significant advancements in the development of improved germplasm. Due to this, algae cultivation may also advance with the use of similar approaches. Similar to traditional agriculture, improvements in mutagenesis, breeding, and genetic engineering have significant roles in algal germplasm enhancement. Domestication of algae can possibly alter their phenotype significantly since the desired characteristics for production are different from those that have changed in the selective pressures of the wild because hypereutrophic aquaculture conditions will sustain genotypes that would not fit in natural environments. Strain engineering and breeding provide the opportunity of stacking desirable traits within one species or a mixture of species. The product type desired, the definition of desirable traits, the specification of harvesting methods and growth, and choice of production organism can influence the needs for supplementary development on a case-by-case basis.
 Presently, researchers have different understandings of the metabolism, genetics, and physiology among the species and genera of algae that might have desirable features for algal biofuel production. Significant difficulties include the need to create genetic technologies for new species that have not been domesticated formerly and that have desirable features for large-scale cultivation. Applying genomic approaches might fast-track the analysis of new strains by identifying the changes in gene expression for a given organism under different conditions and addressing conserved and nonconserved genes between organisms. Those approaches enable the identification of candidate genes that might be pertinent for particular pathways of interest (Flaherty et al., 2011; Karpowicz et al., 2011; Lopez et al., 2011; Weckwerth, 2011). Cryogenic storage methods (e.g. a method used in the Culture Collection of Algae at the University of Texas, UTEX 2012), can also be useful in maintaining germplasm stocks and to replenish pond inocula with a desired genotype after genetic drift of the crop population. Cultured algae (e.g. cultures held for more than 10 years in selective media), have been revealed to have reduced growth and production of unexpected secondary metabolites (Martins et al., 2004).
Presently, researchers have different understandings of the metabolism, genetics, and physiology among the species and genera of algae that might have desirable features for algal biofuel production. Significant difficulties include the need to create genetic technologies for new species that have not been domesticated formerly and that have desirable features for large-scale cultivation. Applying genomic approaches might fast-track the analysis of new strains by identifying the changes in gene expression for a given organism under different conditions and addressing conserved and nonconserved genes between organisms. Those approaches enable the identification of candidate genes that might be pertinent for particular pathways of interest (Flaherty et al., 2011; Karpowicz et al., 2011; Lopez et al., 2011; Weckwerth, 2011). Cryogenic storage methods (e.g. a method used in the Culture Collection of Algae at the University of Texas, UTEX 2012), can also be useful in maintaining germplasm stocks and to replenish pond inocula with a desired genotype after genetic drift of the crop population. Cultured algae (e.g. cultures held for more than 10 years in selective media), have been revealed to have reduced growth and production of unexpected secondary metabolites (Martins et al., 2004).
Presently, there are a few eukaryotic algal species that are readily responsive to genetic engineering or breeding. There are published transformation methods that are well-developed for Phaeodactylum tricornutum and Chlamydomonas reinhardtii. On the other hand, Solazyme seems to depend on the genetically engineered species, Chlorella, for heterotrophic fermentation of algal oils. There are about 30 strains of eukaryotic microalgae that have been transformed with the use of deoxyribonucleic acid (DNA) transfer from Agrobacterium tumefaciens, biolistic bombardment, electroporation, or vigorous mixing with glass beads. However, in many cases the reported transformation have only been fleeting (Radakovits et al., 2010), and these reports have not led to the routine application and adoption for majority of these strains. Nonetheless, these transformations show that developing genetic systems for varied species is conceivable with focused effort.
Genetic manipulation is more straightforward between cyanobacteria compared to eukaryotic algae. This is because prokaryotes are responsive to techniques of bacterial genetics – some species are naturally transformable and take up exogenous DNA without precise intervention (Heidorn, 2011; Ruffing, 2011). Different methods for gene inactivation through the stable expression of transgenes and homologous recombination from plasmids or integrated into the chromosome, are well-known in at least a dozen diverse species (Ducat et al., 2011; Ruffing, 2011). However, the established model organisms have been sustained in the laboratory for several decades and may not be suitable for outdoor cultivation conditions.
For example, the Spirulina species that grow robustly outdoors has been proven to be recalcitrant to manipulation. Despite some reports of transgenic Spirulina (Toyomizu et al., 2001; Kawata et al., 2004), many laboratories have been unsuccessful in achieving stable transformations of the organism. This failure is likely may be due to different restriction endonucleases that specifically cleave foreign DNA (Zhao et al., 2006). Approaches that protect plasmids by methylation, before they are introduced to the cyanobacterium and while they are in an Escherichia coli host by conjugation have enabled genetic technologies for the nitrogen-fixing filamentous strains Anabaena (Nostoc).
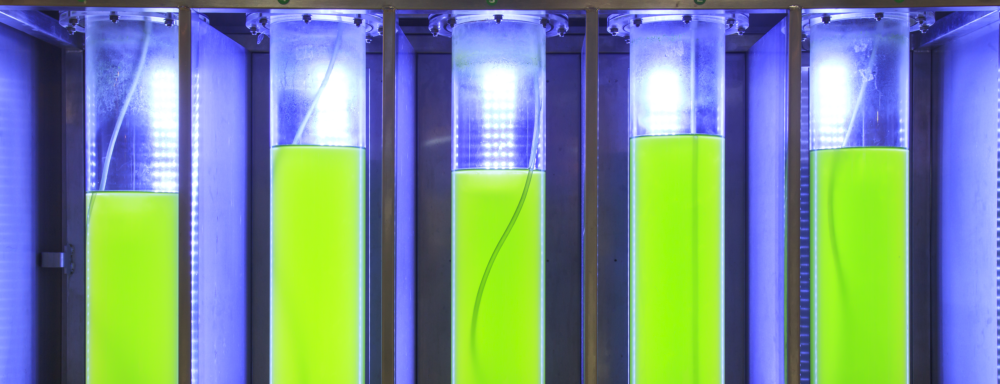
Overview of Algae-Growing Systems
The large-scale and commercial cultivation of microalgae began back in the 1960s. It started with the cultivation of Chlorella in Japan (Tsukuda et al., 1977) and the usage of phytoplankton as a feedstock for animals raised in aquaculture (Duerr et al., 1998). Following that, the Spirulina was harvested from Lake Texcoco in Mexico (Durand-Chastel, 1980) in 1970 and produced in Thailand (Kawaguchi, 1980). In 1980, there were already 46 large-scale facilities operated throughout Asia which produced more than 1,000 kg of microalgae every month (Kawaguchi, 1980). In 2005, the global production of microalgal biomass was estimated to be more than 5,000 dry tonnes, costing more than U.S. $1.25 billion. This excludes the value of processed products (Spolaore et al., 2006). Around 3,000 dry tonnes of Spirulina are produced Japan, China, United States, Myanmar, and India; 2,000 dry tonnes of Chlorella are produced in Japan, Germany, and Taiwan; and 1,200 dry tonnes of Dunaliella salina are produced in the United States, Australia, China, and Israel (Spolaore et al., 2006). In 2008, the global production of microalgal biomass was assessed to be about 9,000 dry tonnes every year (Benemann, 2008).
On top of algal biology and the intended algal products, several factors are taken into consideration when selecting the system to be used for algal cultivation. These factors include the availability and cost of labor, water, nutrients, energy, and land. The climate of the location is taken into consideration as well (Borowitzka, 1992). The characteristics of every cultivation system, including its light utilization efficiency, ability to maintain a unialgal culture, mixing or hydrodynamic characteristics, ease of scaling from laboratory to pilot and commercial scales, and ability to control temperature are also taken into consideration (Borowitzka, 1999). The two general types of algal cultivation systems discussed here closed photobioreactor systems and open-pond systems.
Open-Pond Systems
Presently, a majority of large-scale, commercial microalgal production systems are open-pond systems. This is largely due to ease of scale up and economic factors. Majority of commercial-scale microalgal cultivation operations produce nutraceuticals, not fuel. The quantity of microalgal species that can be cultivated successfully in open-pond systems is limited by the species’ capacity to flourish in predominantly selective environments, while the ponds remain fairly free of protozoan and other algal species pollution (Borowitzka, 1999; Milledge, 2011). For instance, Spirulina is grown at high pH and bicarbonate concentration, Dunaliella salina at high salinity, and Chlorella is grown in a nutrient-rich medium (Borowitzka, 1999; Milledge, 2011).
There are two common types of open-pond systems: raceway ponds and circular ponds. Raceway ponds are built either as a single unit or as a group of continuous that are joined together. A raceway channel enables algae culturing in ponds as deep as 15-40 centimeters. A raceway channel can be built from compacted earth or concrete that might be lined with plastics. An air-lift pump, a paddle wheel, or a propeller operates non-stop to circulate and agitate the mixture in order to avoid algae sedimentation (Becker, 1994; Chen et al., 2009).
On the other hand, circular ponds are round ponds as deep as 30-70 centimeters (Moheimani and Borowitzka, 2006). They are normally agitated with the use of a centrally-pivoted rotating arm. Ponds up to 45 meters in diameter have been operated in Taiwan and Japan (Becker, 1994). Oscillatoria that is cultivated in a circular pond has achieved a productivity of around 15 grams dry weight per m2per day (Sheehan et al., 1998). However, in ponds with diameters greater than 50 meters, mixing efficiency has been shown to be poor (Shen et al., 2009).